Molybdenum Suppliers
 Molybdenum is a refractory metal with great high temperature strength, a very low vapour pressure and low thermal expansion. It has excellent thermal and electrical conductivity up to very high temperatures.
Molybdenum is a refractory metal with great high temperature strength, a very low vapour pressure and low thermal expansion. It has excellent thermal and electrical conductivity up to very high temperatures.
All of our refractory metals such as Molybdenum are supplied by PLANSEE Group of Companies based in Reutte, Austria.
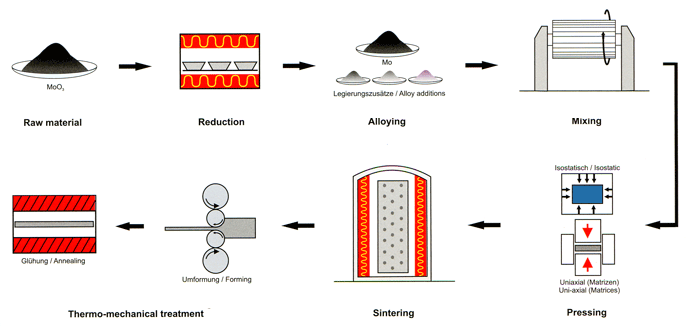
Molybdenum Processing
More information about Molybdenum
Molybdenum is a Group 6 chemical element with the symbol Mo and atomic number 42. The free element, which is a silvery metal, has the sixth-highest melting point of any element. It readily forms hard, stable carbides, and for this reason it is often used in high-strength steel alloys. Molybdenum does not occur as the free metal in nature, but rather in various oxidation states in minerals. Industrially molybdenum compounds are used in high pressure and high temperature applications, as pigments and catalysts.
Molybdenum minerals have long been known, but the element was "discovered" (in the sense of differentiating it as a new entity from minerals salts of other metals) in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm.
Most molybdenum compounds have low water solubility, but the molybdate ion MoO2−4 is soluble and will form if molybdenum-containing minerals are in contact with oxygen and water. Recent theories suggest that the release of oxygen by early life was important in removing molybdenum from minerals into a soluble form in the early oceans, where it was used as a catalyst by single-celled organisms. This sequence may have been important in the history of life, because molybdenum-containing enzymes then became the most important catalysts used by some bacteria to break into atoms the atmospheric molecular nitrogen, allowing biological nitrogen fixation. This, in turn allowed biologically driven nitrogen-fertilization of the oceans, and thus the development of more complex organisms. At least 50 molybdenum-containing enzymes are now known in bacteria and animals, though only the bacterial and cyanobacterial enzymes are involved in nitrogen fixation. Due to the diverse functions of the remainder of the enzymes, molybdenum is a required element for life in higher organisms (eukariotes), though not in all bacteria.
Physical Charateristics
In its pure form, molybdenum is silvery white metal with a Mohs hardness of 5.5. It has a melting point of 2,623 °C (4,753 °F); of the naturally occurring elements, only tantalum, osmium, rhenium, tungsten and carbon have higher melting points.[3] Molybdenum burns only at temperatures above 600 °C (1,112 °F).[4] It has one of the lowest coefficient of thermal expansion among commercially used metals.[5] Tensile strength of molybdenum wires increases about 3 times from about 10 to 30 GPa when their diameter decreases from ~50–100 nm to 10 nm.[6]
Applications
The ability of molybdenum to withstand extreme temperatures without significantly expanding or softening makes it useful in applications that involve intense heat, including the manufacture of aircraft parts, electrical contacts, industrial motors and filaments.[5][27] Molybdenum is also used in alloys for its high corrosion resistance and weldability.[4][20] Molybdenum contributes corrosion resistance to type 316 stainless steel by 'gettering' residual carbon, preventing the formation of chromium carbide at grain boundaries. Most high-strength steel alloys contain 0.25% to 8% molybdenum.[3] Despite such small portions, more than 43,000 tonnes of molybdenum are used as an alloying agent each year in stainless steels, tool steels, cast irons and high-temperature superalloys.[4]
Because of its lower density and more stable price, molybdenum is used instead of tungsten.[4] An example is the 'M' series of high-speed steels such as M2, M4 and M42 as substitution for the 'T' steel series. Molybdenum can be implemented both as an alloying agent and as a flame-resistant coating for other metals. Although its melting point is 2,623 °C (4,753 °F), molybdenum rapidly oxidizes at temperatures above 760 °C (1,400 °F) making it better-suited for use in vacuum environments.[27]
Other molybdenum based alloys have only limited applications. Because of the corrosion resistance against molten zinc, molybdenum and the molybdenum tungsten alloy (70%/30%) are used for piping, stirrers and pump impellers which come into contact with molten zinc.[28]
Molybdenum-99 is a parent radioisotope to the daughter radioisotope technetium-99m, which is used in many medical procedures.[29]
Molybdenum disulfide (MoS2) is used as a solid lubricant and a high-pressure high-temperature (HPHT) antiwear agent. It forms strong films on metallic surfaces and is a common additive to HPHT greases—in case of a catastrophic grease failure, thin layer of molybdenum prevents contact of the lubricated parts.[30] Molybdenum disilicide (MoSi2) is an electrically conducting ceramic with primary use in heating elements operating at temperatures above 1500 °C in air.[31]
Molybdenum trioxide (MoO3) is used as an adhesive between enamels and metals.[17] Lead molybdate (wulfenite) co-precipitated with lead chromate and lead sulfate is a bright-orange pigment used with ceramics and plastics.[32]
Molybdenum powder is used as a fertilizer for some plants, such as cauliflower.[4] It is also used in NO, NO2, NOx analyzers in power plants for pollution controls. At 350 °C (662 °F) the element acts as a catalyst for NO2/NOx to form only NO molecules for consistent readings by infrared light.[33] Ammonium heptamolybdate is used in biological staining procedures.
Source: Wikipedia.org